Cardiovalve Submits CE File for Approval Following Successful TARGET Study Completion
Cardiovalve Submits CE File for Approval Following Successful TARGET Study Completion |
| [17-November-2025] |
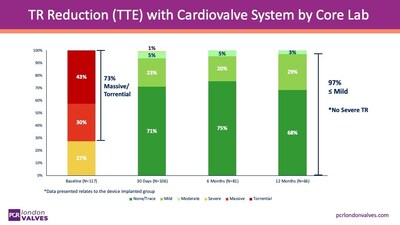
Exciting Data on Cardiovalve Tricuspid Valve Replacement Device Presented at PCR London Valves 2025 meeting. LONDON, Nov. 17, 2025 /PRNewswire/ -- Venus Medtech (Hangzhou) Inc. (2500.HK) today announced that Cardiovalve has submitted the CE technical file for its transcatheter tricuspid valve replacement (TTVR) system to DEKRA, the notified body responsible for CE mark approval in Europe. This milestone follows the successful completion of the TARGET study, which enrolled 150 patients and demonstrated the safety and performance of the Cardiovalve system. The TARGET Study Interim Results : "Safety and Performance of the Cardiovalve Replacement System for Tricuspid Regurgitation" were presented yesterday by Prof. Georg Nickenig, on behalf of the TARGET study investigators, during the Top Late-Breaking Trials session at PCR London valves Main Arena. The TARGET Study followed 150 patients across 30 sites in Europe, the UK, and Canada as part of a prospective, single-arm, open-label, multicenter clinical trial, designed to evaluate the safety and performance of the Cardiovalve TR System. The study interim results, published on Nov.16th,2025, showed that Transcatheter Tricuspid Valve Replacement (TTVR) with the Cardiovalve System effectively eliminated tricuspid regurgitation (TR) in the majority of patients, despite 73% presenting with massive or torrential TR at baseline, demonstrating the clinical effectiveness of the device in patients. In addition to endpoints for efficacy, the procedure was observed to have an acceptable safety profile, with improvements in patients' symptoms by 30 days post-treatment. Patients enrolled in the study will continue to be followed for up to five years. Comprehensive clinical and echocardiographic outcomes, including mortality and heart failure hospitalizations from the full 150-patient cohort, will be presented in the near future. Prof George Nickenig said : "The results of the Cardiovalve study are highly encouraging, demonstrating strong efficacy in tricuspid regurgitation (TR) reduction and a favorable safety profile, particularly with the new device iteration. These findings represent an important step forward, offering hope that patients suffering from TR will soon have access to a novel and effective treatment option." "The submission of the CE file marks a pivotal milestone and brings Cardiovalve closer to delivering transformative therapy for patients suffering from severe mitral and tricuspid regurgitation," said Amir Gross, CEO of Cardiovalve, "This achievement reflects the commitment of a focused team with a shared vision, a team that doesn't just aim; it hits the target. Deep appreciation goes to the investigators, coordinators, clinical partners, and most importantly, the patients and their families for their trust." Lim Hou-Sen, CEO of Venus Medtech, stated: "These promising results mark a significant advancement for patients suffering from tricuspid regurgitation. With the CE submission under review, Cardiovalve is well-positioned to achieve certification and begin commercial rollout of the TR system by 2027." About Cardiovalve A subsidiary of Venus Medtech, Cardiovalve is a pioneer in transcatheter valve replacement technology, standing at the forefront of innovation in structural heart therapy. With more than 150 approved patents, a highly experienced team, and a state-of-the-art manufacturing facility, Cardiovalve is dedicated to providing physicians with next-generation solutions that improve patient outcomes and quality of life—without the need for open-heart surgery. About Venus Medtech Venus Medtech (Hangzhou) Inc. (2500.HK)is a leading innovator in transcatheter heart valve solutions for structural heart disease. The company has developed a comprehensive product pipeline covering all four heart valves — TAVR, TPVR, TMVR, and TTVR —and related accessory products. With global R&D centers in China, the United States, and Israel, the company is committed to providing effective treatment solutions for life-threatening diseases.
SOURCE Venus Medtech (Hangzhou) Inc. | ||
Company Codes: HongKong:02500 |